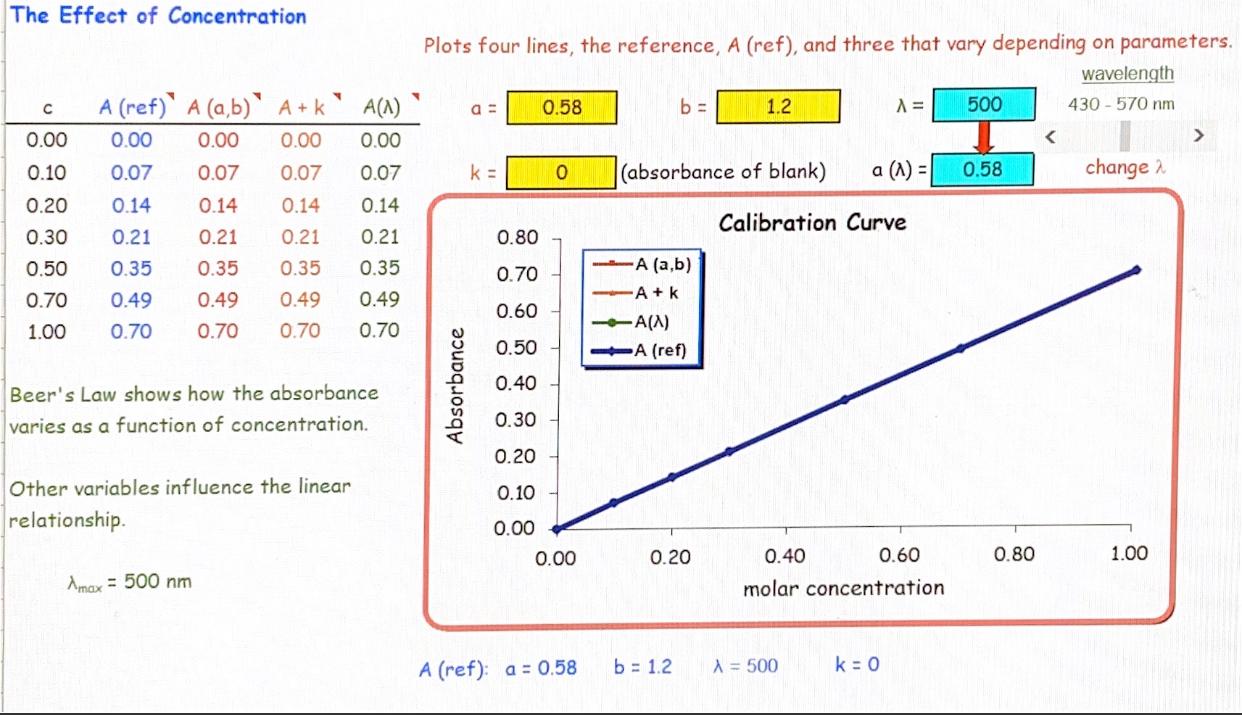
Beer's Law Values . Many compounds absorb ultraviolet (uv) or visible (vis.) light. beer's law states that a chemical solution's concentration is directly proportional to its. As light passes through a sample some of the light will be absorbed by the sample. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can.
from www.chegg.com
beer's law states that a chemical solution's concentration is directly proportional to its. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. Many compounds absorb ultraviolet (uv) or visible (vis.) light. As light passes through a sample some of the light will be absorbed by the sample.
Solved Beer’s law is a linear relationship between
Beer's Law Values since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. Many compounds absorb ultraviolet (uv) or visible (vis.) light. As light passes through a sample some of the light will be absorbed by the sample. beer's law states that a chemical solution's concentration is directly proportional to its. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Values Many compounds absorb ultraviolet (uv) or visible (vis.) light. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known. As light passes through a sample some of the light will be absorbed by the sample.. Beer's Law Values.
From www.youtube.com
BeerLambert law in easy way YouTube Beer's Law Values Many compounds absorb ultraviolet (uv) or visible (vis.) light. As light passes through a sample some of the light will be absorbed by the sample. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can.. Beer's Law Values.
From rrennenextanner.blogspot.com
Beer Lambert Law Ppt rrennenexTanner Beer's Law Values the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. Many compounds absorb ultraviolet (uv) or visible (vis.) light. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. beer's law states that a chemical. Beer's Law Values.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Values Many compounds absorb ultraviolet (uv) or visible (vis.) light. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. equation 8.2.3 and equation 8.2.4, which. Beer's Law Values.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples Beer's Law Values Many compounds absorb ultraviolet (uv) or visible (vis.) light. beer's law states that a chemical solution's concentration is directly proportional to its. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration, path length and molar absorptivity are all. Beer's Law Values.
From lelandchemclub.weebly.com
Leland Chemistry Club Leland Chemistry Club News Beer's Law Values As light passes through a sample some of the light will be absorbed by the sample. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. beer's law states that a chemical solution's concentration. Beer's Law Values.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Values Many compounds absorb ultraviolet (uv) or visible (vis.) light. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. beer's law states that a chemical. Beer's Law Values.
From study.com
How to Find the Absorbance of a Solution Using the BeerLambert Law Beer's Law Values As light passes through a sample some of the light will be absorbed by the sample. beer's law states that a chemical solution's concentration is directly proportional to its. Many compounds absorb ultraviolet (uv) or visible (vis.) light. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a. Beer's Law Values.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Values the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known. As light passes through a sample some of the light will be absorbed by the sample. . Beer's Law Values.
From www.youtube.com
Worked example Calculating concentration using the BeerLambert law Beer's Law Values the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. As light passes through a sample some of the light will be absorbed by the sample. Many compounds absorb ultraviolet (uv) or visible (vis.) light. beer's law states that a chemical solution's concentration. Beer's Law Values.
From hudsontinhoffman.blogspot.com
Beer's Lambert Law Equation HudsontinHoffman Beer's Law Values beer's law states that a chemical solution's concentration is directly proportional to its. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known. Many compounds absorb. Beer's Law Values.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer's Law Values equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known. As light passes through a sample some of the light will be absorbed by the sample. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. . Beer's Law Values.
From chemistrysources.com
قانون بيير (بير) Beer's Law التعريف و المعادلة مصادر الكيمياء Beer's Law Values the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. Many compounds absorb ultraviolet (uv) or visible (vis.) light. As light passes through a sample some. Beer's Law Values.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Values Many compounds absorb ultraviolet (uv) or visible (vis.) light. As light passes through a sample some of the light will be absorbed by the sample. beer's law states that a chemical solution's concentration is directly proportional to its. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known. since the concentration,. Beer's Law Values.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Values since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. beer's law states that a chemical solution's concentration is directly proportional to its. As light passes through a sample some of the light will be absorbed by the sample. Many compounds absorb ultraviolet (uv) or visible (vis.) light. equation 8.2.3. Beer's Law Values.
From www.slideserve.com
PPT Atmospheric Transmission PowerPoint Presentation, free download Beer's Law Values beer's law states that a chemical solution's concentration is directly proportional to its. As light passes through a sample some of the light will be absorbed by the sample. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration,. Beer's Law Values.
From dxokacddk.blob.core.windows.net
Beer's Law Absorption at Ellen Day blog Beer's Law Values since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are. Beer's Law Values.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Values beer's law states that a chemical solution's concentration is directly proportional to its. As light passes through a sample some of the light will be absorbed by the sample. the two laws, beer’s law & lambert’s law, are combined to give a common relation between the absorbance of a solution to its concentration and. since the concentration,. Beer's Law Values.